专注于SF6气体检测的专业化

SF6气体多少度开始液化?
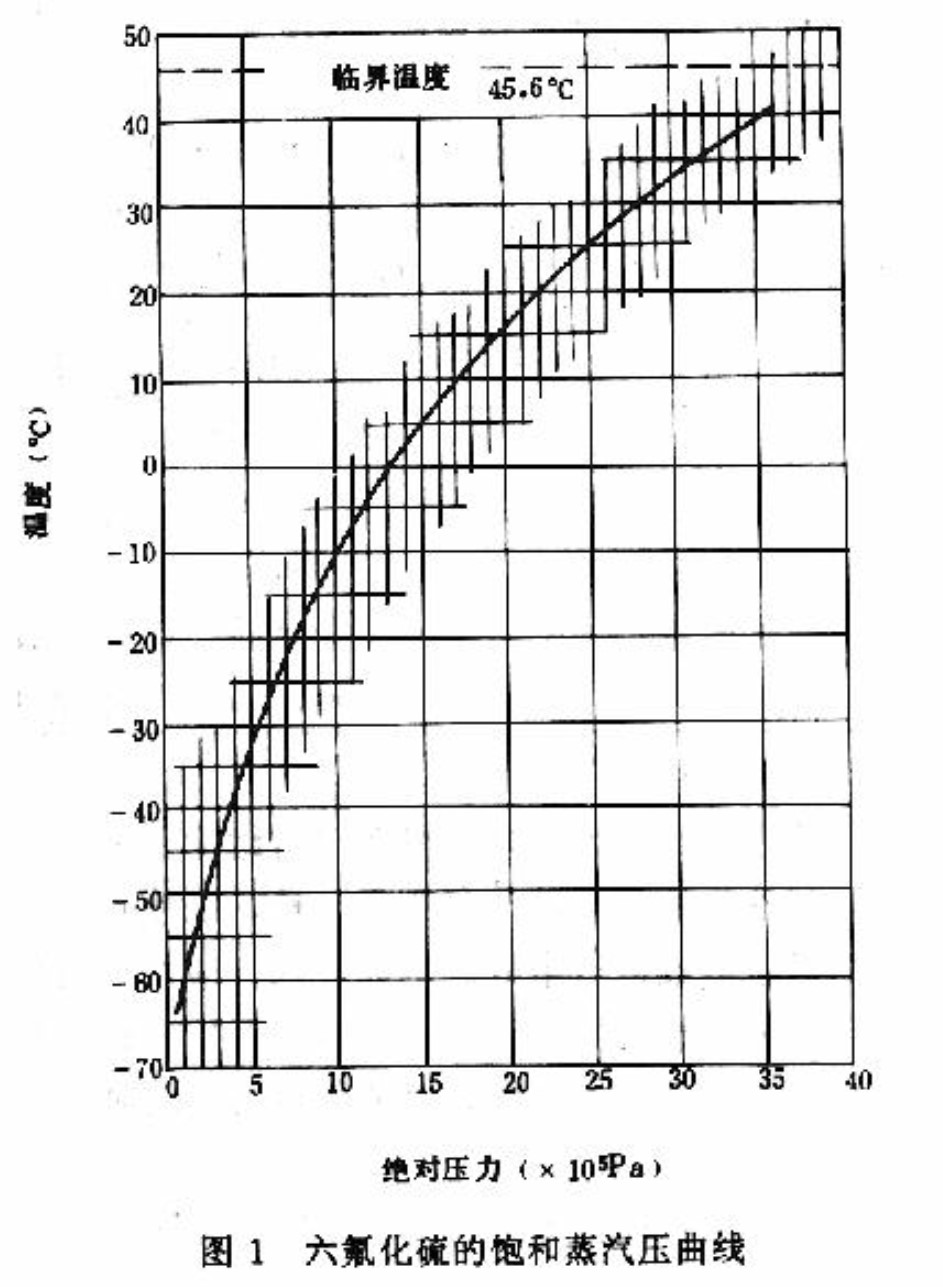
High purity (SF6) sulfur hexafluoride is a gas at normal temperature and pressure, and it is easier to liquefy to liquid under a fixed pressure after the temperature is reduced. When the ambient temperature rises, the corresponding liquefaction pressure rises correspondingly and becomes a gas that is not easy to liquefy. When the temperature exceeds the critical temperature of 45.55 ° C for SF6, it cannot be liquefied.
At present, there are two main recovery and storage principles of high-purity sulfur hexafluoride recovery inflatable device, one of which uses the principle of freezing liquefaction method: That is, in the process of SF6 gas recovery, under a certain SF6 gas pressure, the refrigeration unit is used to reduce the temperature of SF6 gas to the saturated steam temperature under the pressure, SF6 gas begins to liquefy into a liquid, and is stored in a liquid form, and the advantage is that the recovery speed is fast and the liquefaction speed is fast.
The other is the principle of high-pressure liquefaction method, that is, in the process of high-purity sulfur hexafluoride recovery, at the ambient temperature at that time, the compressor is used to increase the pressure of SF6 gas to the saturated steam pressure at that temperature, and SF6 gas begins to be transformed into a liquid and stored in liquid form.
The advantage of sulfur 6 fluoride recovery and purification device is that the system is simple, the disadvantage is that the system working pressure is high, the recovery rate is low, when the SF6 gas temperature (SF6 gas compressed by the compressor temperature can reach 80℃ or more) exceeds the critical temperature of 45.55℃, it can not be liquefied. At this time, the high-temperature and high-pressure SF6 gas can only be converted into a liquid after a period of time when its temperature drops below the critical temperature.
Sulfur hexafluoride, molecular formula SF6, relative molecular weight is 146.06, at normal temperature and pressure for colorless, tasteless, non-toxic, non-corrosive, non-flammable, non-explosive gas, density of about 5 times the air, the standard state density of 6.0886kg/ cubic meter. It is liquid at low temperature and under pressure and becomes a white solid when frozen. The sublimation temperature is -63.9℃, the melting point is -50.8℃, the critical temperature is 45.55℃, and the critical pressure is 3.759MPa. Sulfur hexafluoride has good chemical and thermal stability, excellent electrical insulation and arc extinguishing performance.
SF6 gas liquefaction temperature: it is under one atmospheric pressure (i.e. 0.1MPa), the liquefaction temperature is -62℃; Under the pressure of 1.2MPa, the liquefaction temperature is 0℃. Generally, the SF6 gas pressure charged into the circuit breaker is in the range of 0.35 ~ 0.65MPa (specifically determined by the ambient temperature during inflation), and the liquefaction temperature is -40 ° C. That is, sulfur hexafluoride begins to liquefy at 0.60Mpa at -46℃.
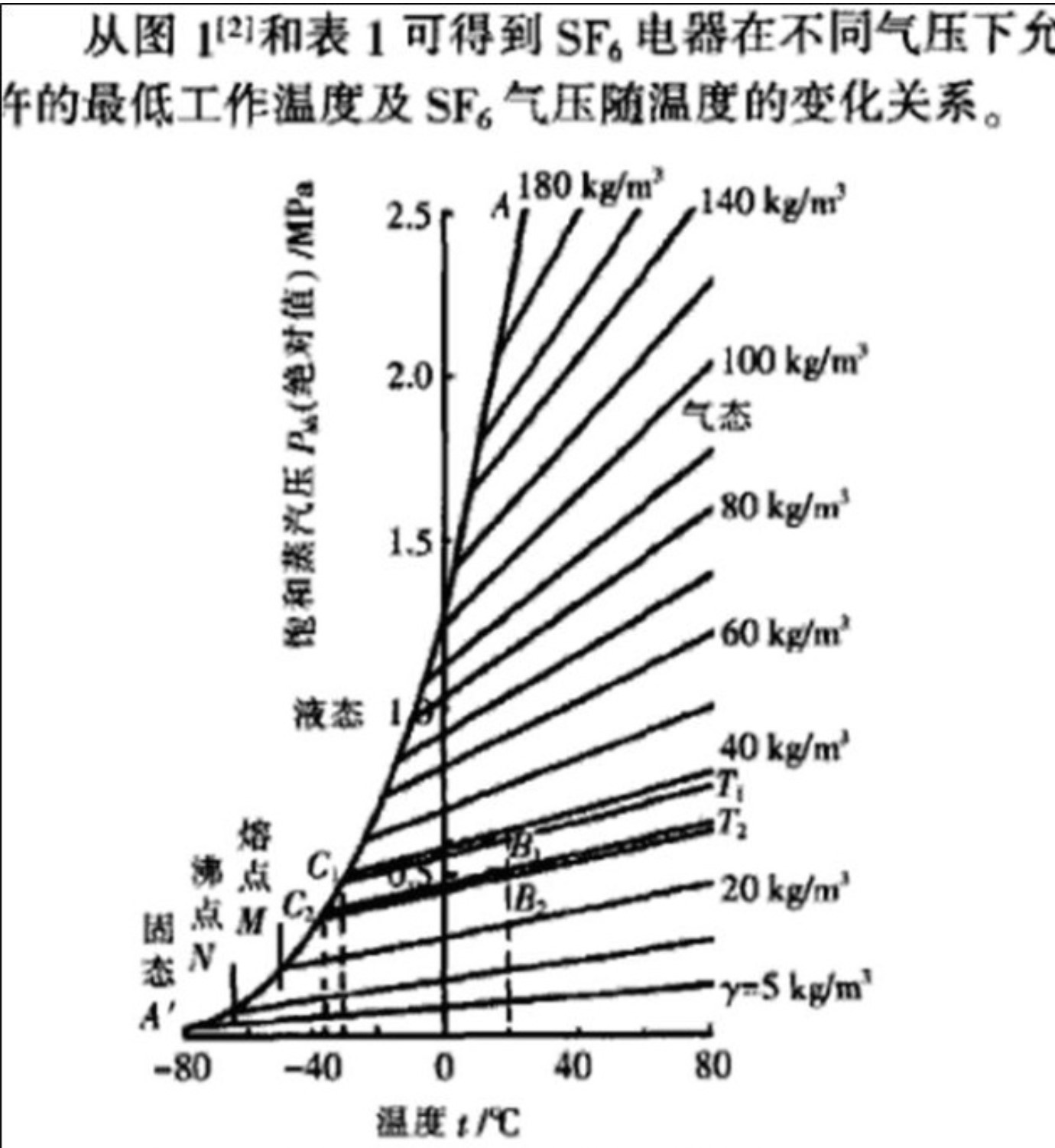
The critical temperature is the highest temperature at which SF6 gas liquefaction occurs. The critical pressure indicates the gas pressure required for liquefaction to occur at this temperature. SF6 can only remain gaseous when the temperature is above 45 degrees, under normal conditions of use, it has the possibility of liquefaction, so SF6 can not be used at low temperatures and too low pressure.
The electrical strength of SF6 is about 2.5 times that of air, and the arc extinguishing ability is more than 100 times that of air, so it has completely replaced insulating oil and compressed air in the category of ultra-high pressure and ultra-high pressure, and has become the circuit breaker arc extinguishing medium.
Some problems in physical and chemical properties of sulfur hexafluoride gas should be used as an insulating medium in engineering practice, which should not only have high electrical strength, but also have good rationalization characteristics. This is the reason why SF6 gas is a highly electronegative gas widely used. The physicochemical properties of SF6 gas in practical applications are described below:
(1) Liquefaction problem
The pressure of the modern SF6 high-voltage circuit breaker is about 0.7Mpa, and the inflation pressure of the rest of the GIS except the circuit breaker is generally not more than 0.45MPa. If the inflation pressure at 20 ° C is 0.75MPa (equivalent to the working pressure commonly used in circuit breakers), the corresponding liquefaction temperature is about -25 ° C, if the inflation pressure at 20 ° C is 0.45MPa, the corresponding liquefaction temperature is 40 ° C, it can be seen that there is generally no liquefaction problem. Only in high cold areas need to use heating measures on the circuit breaker, or use SF6-N2 gas mixture to reduce the liquefaction temperature.
(2) Toxic decomposition
Pure SF6 gas is a non-toxic inert gas, and its compatibility with materials in electrical equipment below 180 degrees Celsius is similar to nitrogen. However, the decomposition of SF6 is toxic and has a corrosive effect on materials, so measures must be taken to ensure the safety of people and equipment.

 EN
EN





 上一条:
上一条: 








 沪公网安备31011802003762
沪公网安备31011802003762
