专注于SF6气体检测的专业化

Why does SF6 gas have arc extinguishing ability?
SF6 molecule has strong electronegativity, which makes SF6 have strong arc extinguishing ability. Because SF6 molecules become negative ions after adsorption of free electrons, negative ions are easy to combine with positive ions to form neutral molecules, so that the electrical conductivity of the arc space quickly disappears. Especially when the arc current is close to zero, this effect is more significant, and SF6 gas blows the arc, making a large number of fresh SF6 molecules constantly in contact with the arc, and the arc extinguishing is more rapid.
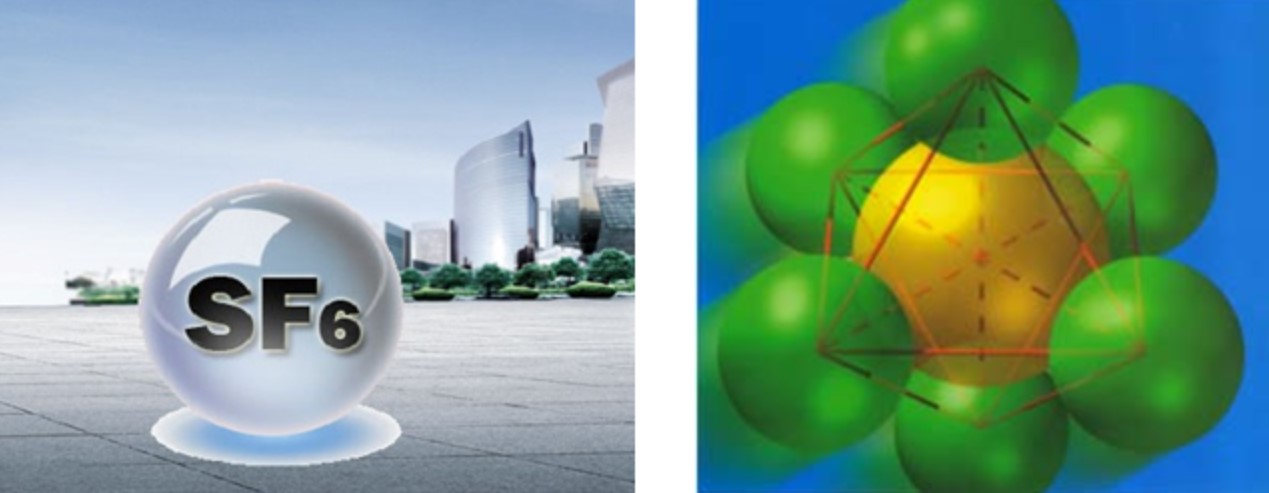
SF6 gas has the following good properties
one Arc column conductivity is high, arc voltage is low, arc column energy is small.
2. When alternating current flows through zero, the dielectric insulation strength of SF6 gas recovers quickly, about 100 times faster than air, that is, its arc extinguishing ability is 100 times higher than that of air.
3. The insulation strength of SF6 gas is high.
The arc extinguishing ability of SF6 gas is stronger than that of ordinary air, and the arc in SF6 is thinner and brighter in the center than that in other media. It shows that low voltage, low extinguishing power and easy to extinguish arc; The center is bright, indicating that the arc center temperature is high, the temperature difference between the surrounding area is large, the natural convection ability is strong, and the surface heat dissipation coefficient is 2.5 times that of the air, which is easy to heat and cool. Because SF6 gas has a large electronegativity, it can absorb a large number of free electrons, and the arc cannot be maintained once it loses free electrons, so the combustion time of the arc in SF6 is shorter than that in oil and air. And no carbon is produced at the contact, and the arc extinguishing ability is reliable.

 EN
EN





 上一条:
上一条: 








 沪公网安备31011802003762
沪公网安备31011802003762
